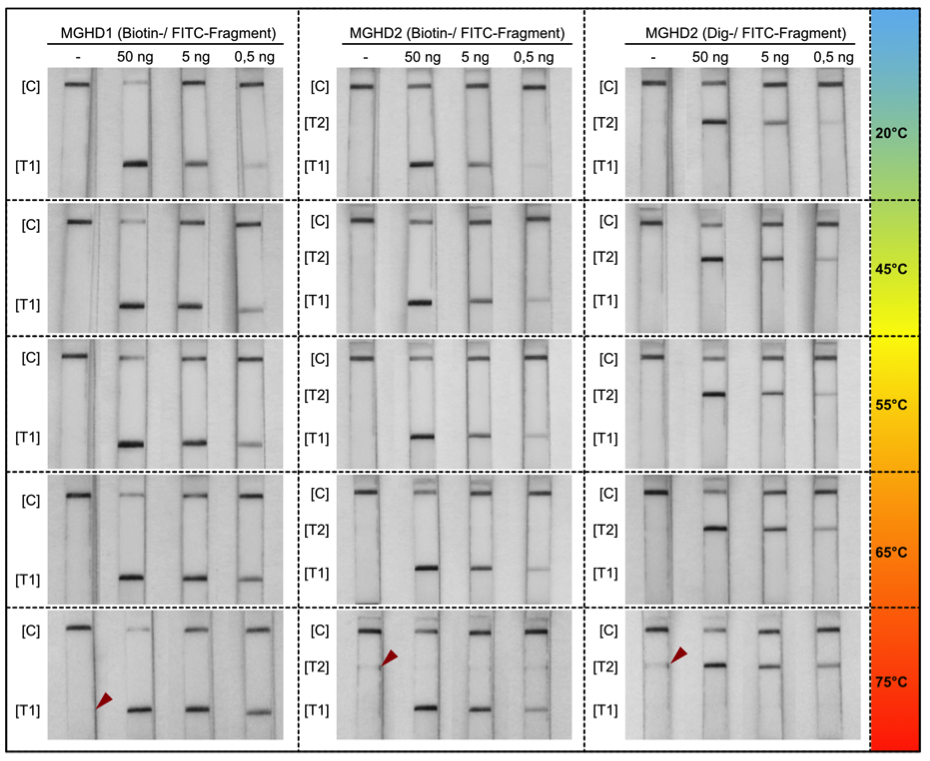
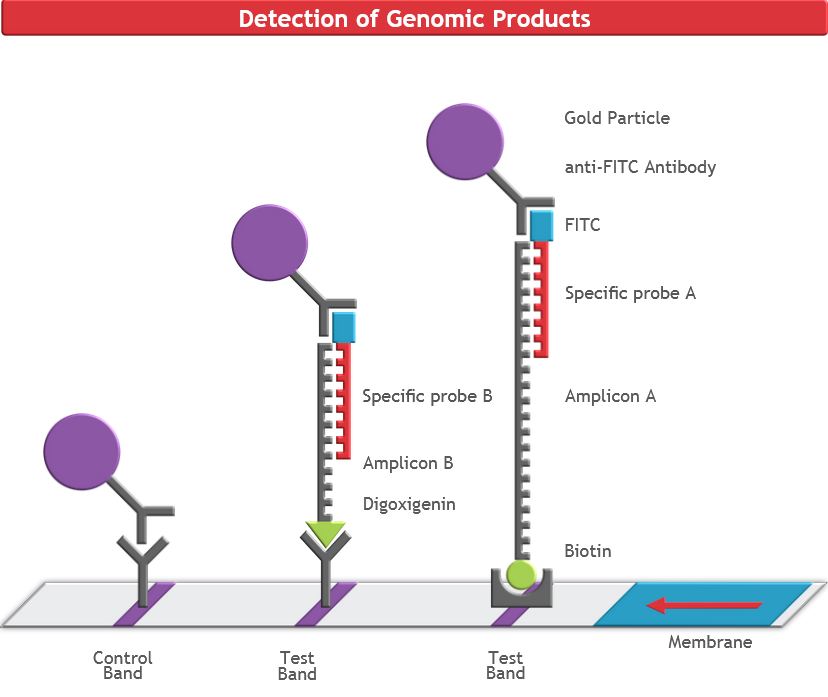
RNA Extraction for COVID-19 Testing
Once the sample arrives in the lab, the RNA needs to be purified from the sample with an extraction kit manually or with an automated liquid handler.
Shortage of RNA Extraction Kits
Just like swabs, there is a dramatic shortage of the reagents required to purify and analyze the viral samples for COVID-19 testing. Once the patient sample arrives in the lab, the RNA needs to be extracted. This process requires a series of chemical buffers that lyse, bind, and purify the viral RNA. Some labs make their own reagents to extract RNA, but most use commercial kits that take hours off the processing time.
With the increasing demand for COVID-19 testing, many labs are struggling to find reagents from their suppliers that are required to process the test. This then delays test results and increases the risk of further spread of the virus as patients may assume the delayed result means they are negative for COVID-19.
The situation has gotten so bad that some scientists have taken to twitter to request donations from other scientific labs. Since the RNA extraction kits are used in a wide range of applications, there were several labs, companies, and research institutes that were not analyzing COVID-19 that could help offset the critically low level of extraction kits.
As one of the largest manufacturers of RNA extraction kits, Zymo Research has increased their manufacturing capabilities to help support the massive need for additional reagents. They will be producing enough of their kits to enable millions of coronavirus tests each month.
COVID-19 Detection
The purified samples contain the host RNA and any COVID-19 RNA (if present). To detect the viral RNA in the sample, a molecular biology technique called real-time reverse transcription polymerase chain reaction(rRT-PCR). This system amplifies the small amount of viral COVID-19 RNA present to thousands of copies, so that may be detected. This makes this assay extremely sensitive.
If there is no virus present within the sample, there will be nothing for the rRT-PCR to amplify and the machine will show a negative result.
Shortage of Reagents
Like with all other supplies in the testing workflow, the detection reagents for the rRT-PCR are also in short supply. There are shortages of the detection buffers, dyes, and enzymes will that dramatically slowdown the time to get results to patients.
Scaling Up
Typically, when a lab begins to process a high number of samples they turn to automated solutions and move away from processing samples by hand. This is done by robotic platforms that greatly increase the throughput of a lab, allowing hundreds or even thousands of samples to be processed in a single day.
Stalled Implementation of Automation
As the testing demand for COVID-19 has grown, it has strained processing labs, and many of these laboratories are looking toward high-throughput automated workflows to keep up with the flood of patient samples that need to be processed. However, these automated workflows usually require lengthy amounts of time before a lab can fully utilize the machine because it has to be set up appropriately.
First, the machine needs to be delivered and installed. Since these are large and sensitive pieces of equipment, this process can take up to a week. Once the robot has been set up, every movement of the robot has to be coded in (or scripted) so that it can manipulate the samples appropriately and isolate the viral RNA. Just like coding an app on your phone, the scripting process is very detailed and time-consuming. Every movement of the robot has to be detailed and entered into the machine: the volume of reagent added at each step, where the reagent is dispensed, how many times the sample should be mixed, and more. Once these commands are correctly entered into the liquid handler, the script has to be tested and then optimized as needed.
From here, the script needs to be validated with test samples to ensure the device can function properly. Once it has been validated, the lab can finally begin processing patient samples on the machine.
This process is time-consuming and arduous for many labs and can take several weeks to complete from the time the device is purchased to the first patient samples being processed on it. In the COVID-19 era, these delays simply hold back labs from testing as many samples as they need to.
The best solution to avoid these delays in implementing automation is to use an automated liquid handler that comes preloaded with scripts that have already been configured and optimized for viral RNA extraction, like the DreamPrep NAP. This device delivers a load-and-go system with a user-friendly interface that dramatically decreases the amount of time needed to begin processing samples. With this device, labs can quickly and easily scale up their operations to accommodate testing for a much higher number of COVID-19 samples.
—
In the world of biology, this workflow is not very complicated. Theoretically, this workflow could function seamlessly with samples flowing in from patients and being processed by the lab with results delivered in 1-2 days. However, the global pandemic has placed a severe strain on the entire testing workflow. Just as with hospitals being overburdened and reaching capacity, testing labs around the world are feeling this same effect. Because of this, there is a severe delay in the implementation and expansion of critical testing services, which adds days to the processing time.
This strain on the testing system is unlike anything the field has ever seen. The industry needs rapid implementation of new solutions to combat the virus from all angles.
With scientists around the world working feverishly to expand production, implement new testing workflows, and streamline processes, the United States is slowly ramping up the amount of tests that can be administered. However, none of these tactics will be effective without the help of the public. Just as the public can flatten the curve for hospitals by staying home, that same effect applies to testing laboratories. With most of the public staying home, they are already helping to resolve the testing bottleneck by decreasing the number of samples that need to be processed. As the world comes together to fight the virus, every single person has a part to play.