Description
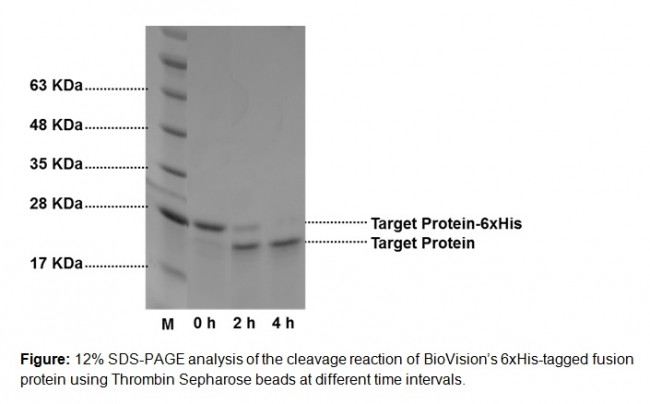
Thrombin Sepharose beads are designed for efficient cleavage of recombinant fusion proteins, containing a thrombin cleavage site (e.g. Leu-Val-Pro-Arg-ll-Gly-Ser; ll: cleavage site), circumventing the need for chromatographic techniques to remove thrombin after cleavage reaction is completed. BioVision’s Thrombin Sepharose is prepared by covalent coupling of bovine thrombin to activated 6% cross-linked Sepharose beads. 15 µl of the slurry is sufficient to cleave >90% of 1 mg of BioVision’s 6xHis-tagged fusion protein in 50 mM Tris buffer, 0.1 M NaCl, pH 8.0 within 4 h at room temperature. These beads can be regenerated for repeat use. The reusability depends on multiple criteria including the usage and handling of the product.
Datasheet
| SKU-Size | 7925-1 (1 ml) 7925-5 (5 ml) 7925-25 (25 ml) |
| Highlights | FORMULATION: 6% cross linked Sepharose beads provided as 50% slurry in pure glycerol. LIGAND DENSITY: 1 mg/ml of the resin. APPLICATIONS: Efficient and convenient cleavage of recombinant fusion proteins containing thrombin-specific cleavage site. PROTOCOL: In order to find the optimum cleavage conditions for a target fusion protein, it is recommended to run preliminary cleavage reactions at a small scale. Successful cleavage with thrombin is dependent upon proper folding of the fusion protein that enables access of the thrombin recognition sequence by the enzyme. Once optimum cleavage conditions are obtained, the reaction can be scaled up to cleave the entire amount of the target fusion protein. The target fusion protein should be purified to homogeneity and dialyzed against 50 mM Tris buffer, 0.1 M NaCl, pH 8.0 before setting up the cleavage reaction. 1) Resuspend the beads by gentle swirling. Do not Vortex. 2) Aliquot 15 µl of the suspended slurry and add to 1 mg of the fusion protein in an Eppendorf tube. The recommended concentration of target fusion protein is 1 mg/ml. 3) Mix gently by inverting the tube (do not vortex) and gently shake on a rotary shaker at room temperature. 4) At regular time intervals spin down the tube to aliquot a test sample and freeze it immediately. At the end of the reaction, analyze the samples by SDS-PAGE. Recovery of the cleaved target protein: 1) After the fusion protein is completely cleaved, spin down the reaction mixture for 2-3 min at 5000 rpm. 2) Remove the supernatant and wash the beads with 0.2 ml of 50 mM Tris buffer, pH 8. 3) Repeat steps 1 and 2 to maximize the recovery of the target protein and its cleaved fragment. Further chromatography may be necessary to remove the cleaved fragments from the target protein. |
|---|---|
| Storage Conditions | -20°C |
| Shipping Conditions | Gel Pack |
| USAGE | For Research Use Only! Not For Use in Humans. |
FAQ
-
 We're here to help
We're here to help
Get expert recommendations for common problems or connect directly with an on staff expert for technical assistance related to applications, equipment and general product use.
Contact Tech Support
 High Quality Guaranteed Product
High Quality Guaranteed Product
Our products such as Elisa, Antibodies, Proteins, Peptides and sequencing kits are covered by Biolinkk quality warranty and will work as described in datasheet, a free replacement or money back is guaranteed if does not perform according to datasheet.
Learn More