IRVINE, Calif., (July 14, 2022) — Zymo Research, in collaboration with the Institute of Virology, University Medical Center Freiburg, Germany, demonstrated data showing their DNA/RNA Shield inactivating transport medium (ITM) completely inactivated recent Monkeypox virus isolates.
This data comes at a critical time as Monkeypox cases continue to climb worldwide and scientists are seeking methods to safely collect and transport samples without compromising the genetic integrity. Monkeypox testing is primarily performed via PCR from a swabbed lesion.
The DNA/RNA Shield product line includes sample collection, preservation, and transportation devices for specimens used in research and infectious disease testing workflows. DNA/RNA Shield has enabled researchers to conduct infectious disease research and testing over the past decade, including throughout the COVID-19 pandemic.
Zymo Research offers several devices that can be filled with DNA/RNA Shield, including the popular SafeCollect Swab Collection Kit. The SafeCollect Swab Collection Kits contain a collection swab and a patented tube that features a safety seal to prevent accidental spillage, contact, and/or ingestion of the sample stabilization medium, making it ideal for at-home sample collection.
Learn more about Zymo Research’s DNA/RNA Shield SafeCollect Sample Collection Kits. here
View datasheet
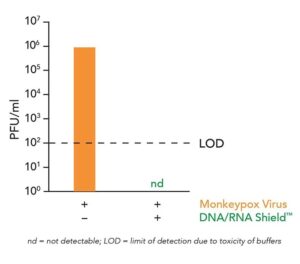
A viral plaque assay demonstrating the inactivation of Monkeypox virus after exposure to Zymo Research’s DNA/RNA Shield reagent.