Explore by Target
Secondary Antibodies
Secondary antibodies bind to primary antibodies and are commonly used to detect and visualise a primary antibody, which is bound to its protein of interest, in applications like western blotting or immunocytochemistry. Multiple secondary antibodies can bind to the same primary antibody, increasing the sensitivity and amplifying the signal. Secondary antibodies are commonly conjugated with reporter molecules, either enzymes like HRP or fluorophores like APC, to enable the fluorescent, colorimetric, or chemiluminescent detection of primary antibodies.
Secondary antibodies are raised against the host species and isotype of the primary antibody. For example, if you used a polyclonal primary antibody raised in goat, you will require an Anti-Goat IgG (Heavy & Light chains) secondary antibody raised in a different species.
Explore our comprehensive portfolio of conjugated and unconjugated secondary antibodies for the fluorescent, colorimetric, and chemiluminescent detection of primary antibodies in a diverse range of applications, including: flow cytometry, immunohistochemistry, immunofluroescence, immunocytochemistry, and western blotting.
Categories
Technical Information
Enzymes, like Horseradish Peroxidase (HRP) or Alkaline Phosphatase (AP), can conjugate to an antibody via covalent linkages. Enzyme conjugated antibodies are used in conjunction with a substrate, either fluorogenic, colorimetric, or chemiluminescent, to create a detectable signal. The non-detectable, soluble substrate is converted by the enzyme into a detectable (and normally insoluble) form. Enzyme conjugated antibodies enable signal amplification; as the enzyme is able to convert many molecules of the substrate, the length of time the reaction is allowed to continue determines the strength of the signal.
Horseradish Peroxidase
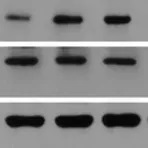
Horseradish peroxidase (HRP) is an enzyme derived from the root of Armoracia rusticana (a.k.a. the horseradish plant) that is commonly used as a reporter protein. HRP conjugated secondary antibodies can either be used for colorimetric detection, where the HRP catalyses the conversion of a chromogenic substrate to a coloured precipitate, or chemiluminescent detection, where the HRP produces a signal by oxidising a chemiluminescent substrate to a form which emits light.
Multiple HRP molecules can bind to a single secondary antibody, as such, HRP conjugated secondary antibodies can be utilised for signal enhancement and enable detection of proteins expressed at low levels. HRP also has a high turnover rate which enables HRP conjugated secondary antibodies to generate a strong signal in a short amount of time (often within five minutes).
Alkaline Phosphatase
Alkaline phosphatase (AP) conjugated secondary antibodies are commonly used for ELISA and western blotting. They are also sometimes used for immunohistochemistry, however, their large size may limit penetration into tissues. It is worth noting, that whilst HRP generates a maximum signal quickly, often within five minutes, the signal from AP gradually increases and peaks at around an hour. AP generates a very stable signal which can last several days; this is particularly useful if multiple exposures are needed, especially over a matter of days.
Trying to decide between HRP and AP for your blot?
Use our table below to help with the decision.
| AP | HRP |
|---|---|
| Sensitive | Sensitive |
| Long lasting signal (24-48 hours) | Quick max signal (~5 minutes) |
| Useful for multiple exposures | Inhibited by azides |
| Inhibited by phosphate buffers | Economical |
Biotin
Biotin is a small, water-soluble B vitamin that can be bound by avidin, NeutrAvidin, and streptavidin with high affinity. Due to its small size, binding with biotin does not typically affect a protein’s biological activity. Multiple biotin molecules can conjugate to a single secondary antibody, as such, biotin conjugated secondary antibodies can be utilised for signal enhancement and enable detection of proteins expressed at low levels. Visualisation happens through biotin / streptavidin interaction whereby the streptavidin is bound to either HRP or a fluorescent probe. By utilising a biotin conjugated secondary antibody, the same secondary antibody can be used in multiple applications simply by switching the streptavidin used.
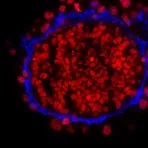
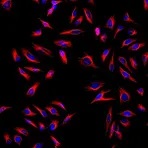
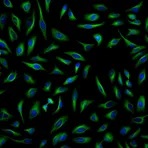
Fluorescent-dye conjugated secondary antibodies allow for a brighter signal and provide the ability to distinguish between multiple proteins in a single sample, as such, they are a valuable tool for identifying proteins in immunocytochemistry, western blotting, immunofluorescence, immunohistochemistry, and many more applications. Common fluorescent dyes include the Alexa Fluor range, AMCA, Cy3, FITC, PE, and TRITC.
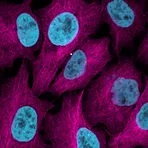
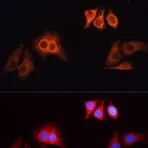
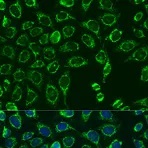
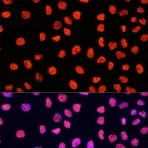
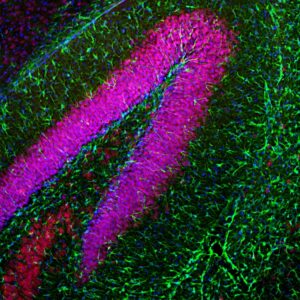
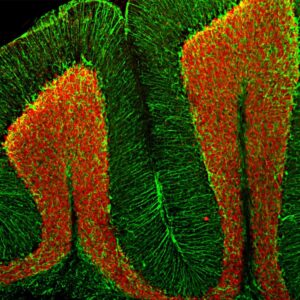
Examples of fluorescent detection:
An antibody is a “Y-shaped” glycoprotein that is capable of binding to specific antigens. Each antibody is composed of four polypeptide chains, two identical heavy chains and two identical light chains, which vary in sequence and length between species and between isotype classes. An antibody’s structure can be broken down into: two F(ab) regions, the top sections of the “Y” which contain the variable region which binds specifically to a particular epitope on the antigen; a hinge region; and an Fc region, the bottom of the “Y” which provides a binding site for endogenous Fc receptors (and secondary antibodies).
In mammals, antibodies are classified into five main classes or isotypes according to the heavy chain they contain. These are: IgA (alpha), IgD (delta), IgE (epsilon), IgG (gamma), and IgM (mu). Each class differs in the sequence of constant domains, the number of constant domains, the hinge structure, and the valency of the antibody. The light chains of an antibody are classified as either kappa or lambda based on their polypeptide sequence. Typically, the two light chains in an individual antibody are the same type.
Whole antibodies can be digested by papain or pepsin to form F(ab), F(ab’) and F(ab’)2 fragment antibodies, which have no Fc portion or a significantly reduced Fc portion. The structures of F(ab), F(ab’) and F(ab’)2 fragments differ: F(ab) fragments are single antigen-binding F(ab) portions, F(ab’) fragments are single F(ab) portions with the hinge region present, and F(ab’)2 fragments are two F(ab) portions linked via the hinge region. F(ab), F(ab’) and F(ab’)2 fragment secondary antibodies are used to prevent the non-specific binding between the Fc region of an antibody and the Fc receptor on a cell.
Antibody Classes and Subclasses
| Immunoglobulin Classes | IgA | IgD | IgE | IgG | IgM | |
|---|---|---|---|---|---|---|
| IgG Subclasses – Goat | IgG1 | IgG2 | ||||
| IgG Subclasses – Human | IgG1 | IgG2 | IgG3 | IgG4 | IgA1 | IgA2 |
| IgG Subclasses – Mouse | IgG1 | IgG2a | IgG2b | IgG3 | ||
| IgG Subclasses – Rat | IgG1 | IgG2a | IgG2b | IgG2c | ||
| Light Chains | Kappa | Lambda | ||||
| Heavy Chains | Alpha | Delta | Epsilon | Gamma | Mu |