In general, two sets of primers (two outer and two inner primers) are used to identify six regions on the target gene (high specificity compared to PCR). LAMP uses Bst DNA polymerase large fragment, which has a high strand displacement activity. Compared to PCR, LAMP is very rapid (1 h).
RPA – Recombinase Polymerase Amplification
RPA is as specific as PCR amplification but much faster and can be done at temperatures between 37 and 42°C in just 10 minutes. RPA uses a recombinase, a single-stranded DNA-binding protein (SSB) and a polymerase. The recombinase pairs the primers to the homologue target DNA sequences. SSB stabilizes the resulting D-loop. DNA synthesis is initiated by the DNA polymerase.
LFD – Lateral Flow Dipstick – HybriDetect
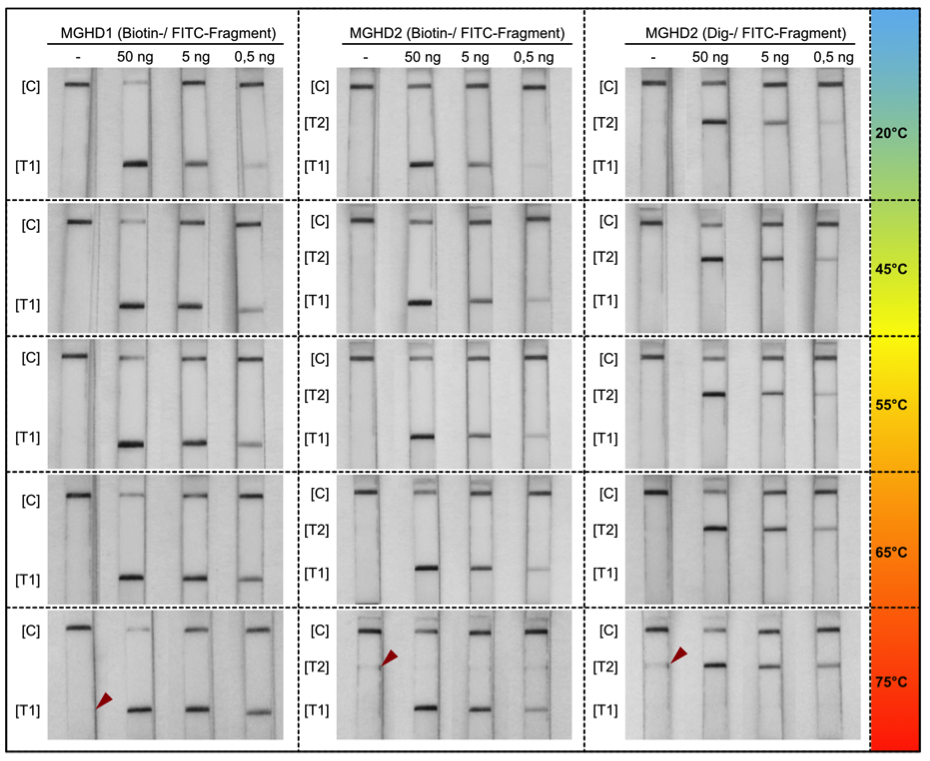
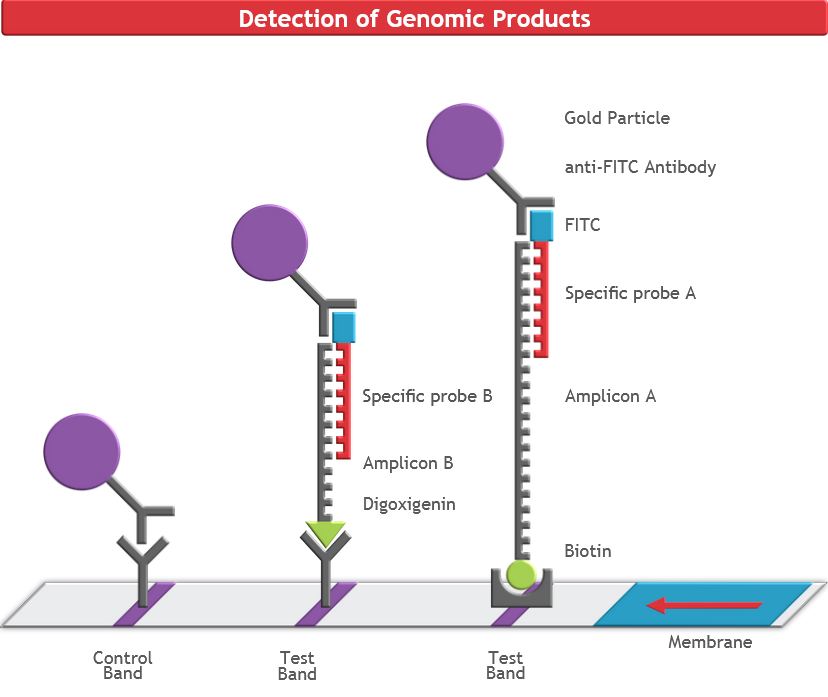
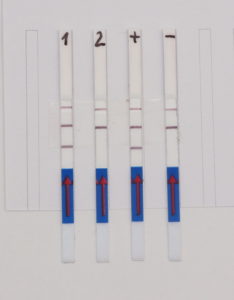
The HybriDetect is a lateral flow dipstick (LFD), which is able to detect different molecules, such as gene amplification products, proteins and antibodies. A commonly used application for our test is to detect gene amplification products resulting from PCR, LAMP or RPA. Therefore, labeled primers must be used during the amplification step, so that the resulting DNA fragments are labeled and can be detected by the HybriDetect dipstick. Results can be reported within 5 minutes after lines become visible on the test Strip. HybriDetect is working with aqueous solution and does not contain toxic reagents such as EthBr.
Detecting Viruses – Applications
Infectious spleen and kidney necrosis virus (ISKNV) detection
read full article
Ding et. al. developes a LAMP combined with the Milenia HybdriDetect 2T Lateral Flow Dipstick (LFD) for the detection of ISKNV (Infectious spleen and kidney necrosis virus), one of the most important pathogens in aquaculture, especially in China. The ISKNV causes high mortality in many freshwater and marine fish. The authors developed a test with a detection limit of 10 copies for the large cytoplasmic dsDNA virus. The method is rapid, sensitive, simple and has a high economic impact. The sensitivity was 1 000 fold higher than in comparable methods , like LAMP-AGE (4).
Cyprinid herpes virus 2 (CyHC-2) detection
read full article
CyHV-2 causes Herpesviral heamoatopoietic necrosis (HVHN) in carp aquaculture and is responsible for huge economic losses in China, USA and Australia. There is no effective prevention and the disease causes mortality up to 100 %. It is crucial, that HVHN is detected in a very early stage of the disease. Wang et al developed a RPA combined with our HybriDetect which can be done in just 15 minutes at 38 °C. The method is 100 times more sensitive than others and the authors couldn’t detect any cross reactions with other aquatic viruses (3).
Cybrinid herpes virus 3 (CyHC-3) and Koi Herpes Virus (KHV) detection
read full article
A LAMP-LFD (HybriDetect) method for the specific detection of cybrinid herpes virus 3 (CyHC-3) and Koi Herpes Virus (KHV) was invented by Soliman and El-Matbouli. This method can detect amounts of 10 fg DNA (30 copies vg) within one hour. It is much faster than the commonly used PCR (3 hours) and 10 to 100 fold more sensitive (5).
Detection and Differentiation of Carp oedema virus (CEV) and koi herpes virus (KHV)
read full article
With the current tests on the market, it is often difficult to determine between CEV and KHV, which both are common carp viruses. In 2017 a rapid and accurate tool was invented by Soliman and Matbouli to detect and differentiate CEV and KHV. The authors used RPA combined with HybriDetect (LFD) in their new assay which is very fast (60 min) compared to current methods (10 and 7 hours). The test can be used in field situation to reduce spread of the viruses (2).
Spring Viremia of Carp Virus (SVCV) Detection
read full article
SVCV is a cyprinid pathogenic virus, which usually needs to be detected in a lab. Most virus outbreaks can be seen in fishery banks. The invented detection method (LAMP with HybriDetect) is suitable for field-detection in aquaculture and can detect up to 860 fg DNA. The authors claim, that a rapid and accurate diagnosis of the virus is vital to prevent the spread of the virus and to minimize economic losses. Many commonly used techniques are time consuming or show cross reactions with other viruses; therefore a simple, but still accurate method like LAMP-LFD is needed (1).
Shrimp Taura Syndome Virus detection
read full article
Kiatpathomchai et al also used a combination of LAMP with LFD to detect the Shrimp RNA Virus Taura Syndrome Virus (TSV). This virus has a big economic impact regarding shrimp farming. The method developed by the authors is very quick (total assay about 70 min.). The sensitivity is comparable to other commonly used methods for RT-PCR detection of TSV (6).
Advantages
In summary the combination of LAMP or RPA combined with HybriDetect (LFD) Shows a number of advantages:
- * Equivalent or even better sensitivity compared to commonly used PCR methods
* Much faster
* No cross reactions to other aquatic viruses
* Field-application
* Nearly no equipment/machines needed
* Cost effective
Literature
- Comparison of Three Terminal Detection Methods Based on Loop Mediated Isothermal Amplification (LAMP) Assay for Spring Viremia of Carp Virus (SVCV). (2019). Turkish Journal of Fisheries and Aquatic Sciences, 19(9), 805–816Soliman, H., & El-Matbouli, M. (2018)
- Rapid detection and differentiation of carp oedema virus and cyprinid herpes virus-3 in koi and common carp. Journal of Fish Diseases, 41(5), 761–772. https://doi.org/10.1111/jfd.12774
- Wang, H., Sun, M., Xu, D., Podok, P., Xie, J., Jiang, Y., & Lu, L. (2018). Rapid visual detection of cyprinid herpesvirus 2 by recombinase polymerase amplification combined with a lateral flow dipstick. Journal of Fish Diseases, 41(8), 1201–1206. https://doi.org/10.1111/jfd.12808
- Ding, W. C., Chen, J., Shi, Y. H., Lu, X. J., & Li, M. Y. (2010). Rapid and sensitive detection of infectious spleen and kidney necrosis virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. Archives of Virology, 155(3), 385–389. https://doi.org/10.1007/s00705-010-0593-4
- Soliman, H., & El-Matbouli, M. (2010). Loop mediated isothermal amplification combined with nucleic acid lateral flow strip for diagnosis of cyprinid herpes virus-3. Molecular and Cellular Probes, 24(1), 38–43. https://doi.org/10.1016/j.mcp.2009.09.002
- Kiatpathomchai, W., Jaroenram, W., Arunrut, N., Jitrapakdee, S., & Flegel, T. W. (2008). Shrimp Taura syndrome virus detection by reverse transcription loop-mediated isothermal amplification combined with a lateral flow dipstick. Journal of Virological Methods, 153(2), 214–217. https://doi.org/10.1016/j.jviromet.2008.06.025